Choosing is essential to delivering vaccines to the outbreak’s epicenter, the Democratic Republic of the Congo.
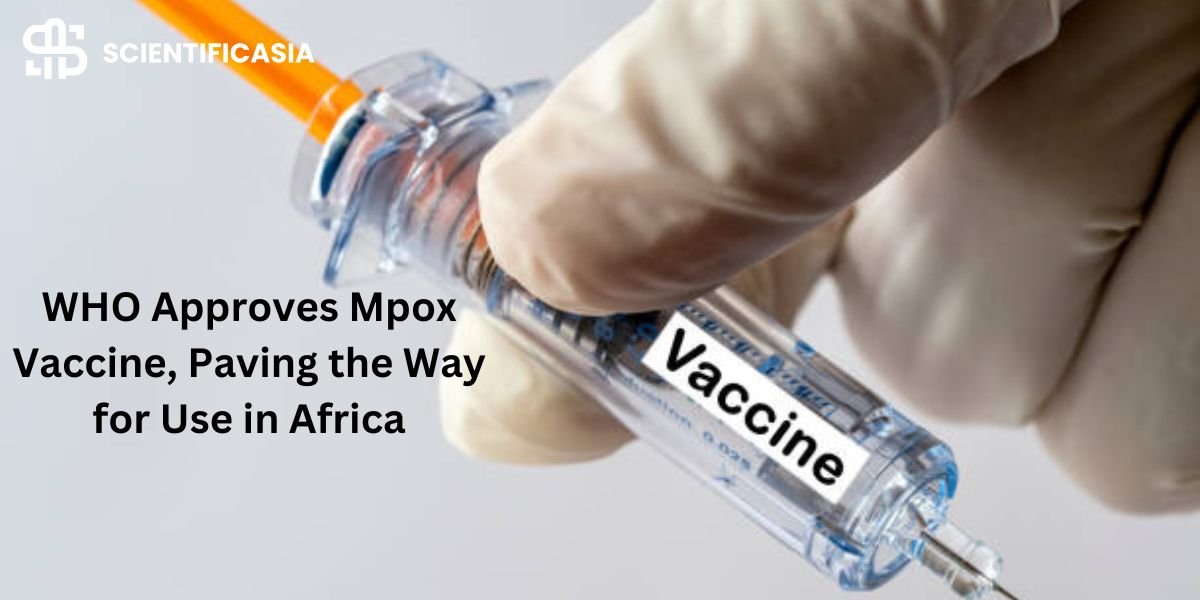
The CEO of the business that manufactures the vaccine was taken aback when the World Health Organization authorized the first vaccination to prevent mpox. The decision was made so quickly on Friday.
Following a global mpox outbreak in 2022, the vaccine, produced by the Danish company Bavarian Nordic, has been licensed by regulatory bodies in Europe, the US, and other high-income nations.
However, through a procedure known as prequalification, low- and middle-income nations depend on the World Health Organization (WHO) to identify which medications, vaccines, and medical technology are safe and effective uses of their limited health funds. Up until this point, the WHO had declined to take any action.
Since announcing a global public health emergency for mpox last month, the World Health Organization has faced mounting criticism for failing to provide a vaccine with the prequalification stamp of approval, or a more provisional type of certification known as an emergency use authorization. In 2023, Bavarian Nordic sent the World Health Organization the vaccine’s safety and efficacy data, known as JYNNEOS.
The World Health Organization had justified its sluggish review process by arguing that the vaccine, along with two other vaccines that have been used to prevent mpox, was initially intended to treat smallpox, and administering it in low-resource settings like Central Africa would involve different considerations than those about its use in high-income nations.
However, the W.H.O. abruptly announced Friday morning that it approved the injection.
The director general of the World Health Organization, Dr. Tedros Adhanom Ghebreyesus, stated in a statement that “this first prequalification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa and in the future.”
Chief executive Paul Chaplin of Bavarian Nordic stated that he was one of the many people who had been taken by surprise.
“I’m not sure exactly how, but we’ve arrived eventually,” he remarked. It’s excellent news, though. It will greatly simplify the regulatory process.
Since it was discovered for the first time in the Democratic Republic of the Congo over 50 years ago, mpox has been endemic there. People in the Congo have continued to contract the virus even if the global spread that began in 2022 slowed down in 2023. In 2023, a new and sexually transmissible strain of the virus was discovered there. This year, there have been over 21,000 suspected instances of mpox, with 700 reported deaths.
Last week, over 245,000 donated shots from the US, the EU, and Bavarian Nordic started to arrive in Kinshasa, the country’s capital. By October 2, the Congolese government had stated that it intended to start distributing them.
A scheduled meeting of a World Health Organization committee to assess the vaccine for emergency use is scheduled for next week, but the Jynneos prequalification decision took precedence.
The World Health Organization’s decision to approve the vaccine at this time, according to Prashant Yadav, a professor of technology and operations management at the French business school INSEAD and an authority on health technology supply chains, was both unexpected and commendable.
“I applaud them for doing this; it’s not routine for them to do such an expedited approval,” the speaker stated.
African health ministers have voiced dissatisfaction with the World Health Organization’s slow reaction, citing the Africa Centers for Disease Control and Prevention’s early declaration of a public health emergency related to mpox.
The World Health Organization (WHO) authorized the vaccine for adults on Friday, but it could also be administered for children and adolescents under the age of 18 at the discretion of medical professionals. UNICEF reports that children have been affected by mpox more than half of the time in the Congo this year. In August, the World Health Organization’s expert committee on immunization declared that, for children who were at high risk of exposure, the advantages of the vaccine outweighed the risks.
The World Health Organization (W.H.O.) directed Gavi, a global organization that provides funding for vaccines to low-income nations, and UNICEF, the agency that purchases the doses, to release a tender for mpox vaccines in late August, even before its experts had had a chance to review the emergency use license.
According to Mr. Chaplin, Bavarian Nordic put in a bid and might provide two million vaccination doses this year and an extra 11 million in 2025. Up to 10 million doses of the vaccine, according to the Africa C.D.C., may be needed to contain the outbreak that has spread to over a dozen African nations, including Burundi and Uganda, which have never before reported a mpox case.
For optimal protection, the vaccination needs to be administered in two doses; however, the World Health Organization advised considering the use of a single shot in emergency scenarios if supplies are limited. In 2022, Bavarian Nordic charged higher-income nations approximately $115 for each shot; Mr. Chaplin, the CEO, stated that Gavi’s pricing would be determined by volume.
Gavi is under pressure to place an order big enough for African nations to have an adequate supply of the vaccine as it has a designated “first response fund” of money earmarked for buying vaccinations in situations like this one.
CDC reports obesity rates exceed 20% in all U.S. states
Morocco reports its first mpox case in the ongoing outbreak
One problem is getting supplies to the countries. The manufacture of the shots is only the first step in trying to guard against the virus; these vaccines involve complicated logistics of transportation, temperature control, personnel training, and community education.
“Translating vaccine deliveries into countries into vaccinations is an important focus now, even though we remain in a supply-constrained environment,” stated Derrick Sim, director of Gavi’s vaccine markets and health security. “One of the lessons learned from the COVID-19 pandemic is that the timing of a country’s vaccination programs must coincide with the supply of vaccines.”
The indemnity challenge will be different. Although Bavarian Nordic is insured against liability for adult vaccination use, off-label use of the vaccine in children is not covered by this insurance.
“The emergency use authorization for each country would need to include use in children,” Mr. Chaplin stated. “Or the company using the product without a label would have to bear the consequences.”
Mass General Brigham study links high doses of Adderall to increased risk of psychosis and mania